First question!
Is radical Nucleophilic or Electrophilic species?
We know that radicals are often considered electron-deficient species that don’t satisfy the octet rule. However, Electron Deficiency ≠ Electrophilicity. In fact, radicals can exhibit either nucleophilic or electrophilic behavior, depending on their tendency to target sites of higher or lower electron density. Radical polarity matching has been one of the most critical aspects of modern radical reaction kinetics research, greatly influencing radicals’ reactivity and selectivity. Recognizing these factors helps us understand, control and design radical reactions
Frontier molecular orbital theory offers deeper insight into radical behavior. The SOMO (Singly Occupied Molecular Orbital) of a radical can interact with both the HOMO and LUMO of a reactant, lowering the transition state energy in both cases. Interaction with the LUMO clearly reduces energy, but the same is true for interaction with the HOMO.
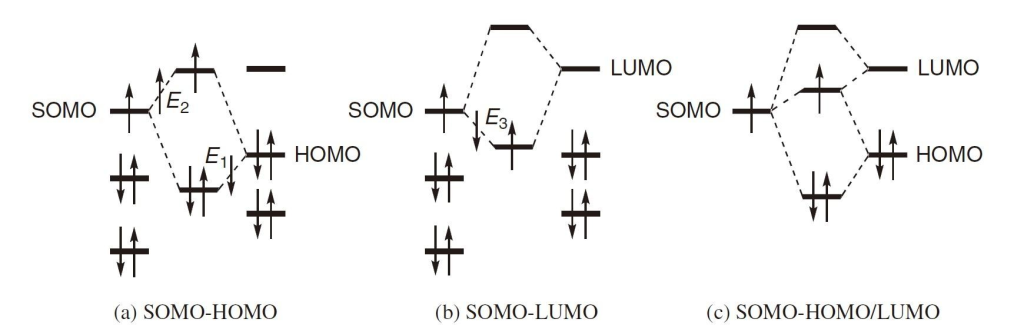
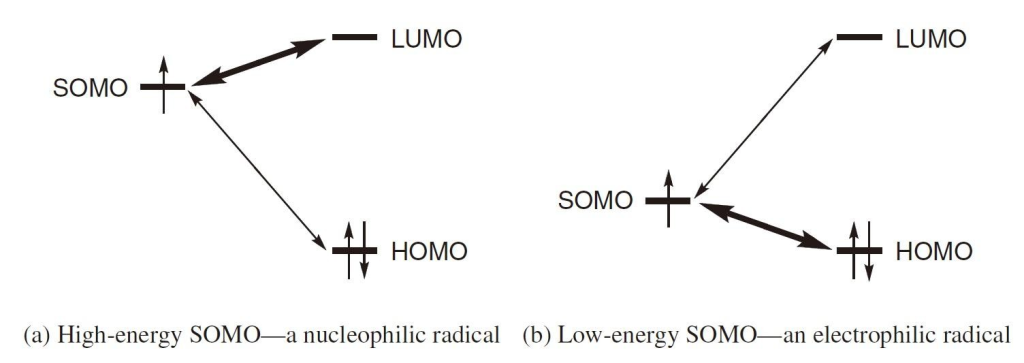
Radicals with high-energy SOMOs tend to react quickly with molecules that have low-energy LUMOs, exhibiting characteristics of nucleophiles. Conversely, radicals with low-energy SOMOs readily react with high-energy HOMOs, behaving like electrophiles. Therefore, the former are classified as nucleophilic radicals, while the latter are considered electrophilic radicals.
Application
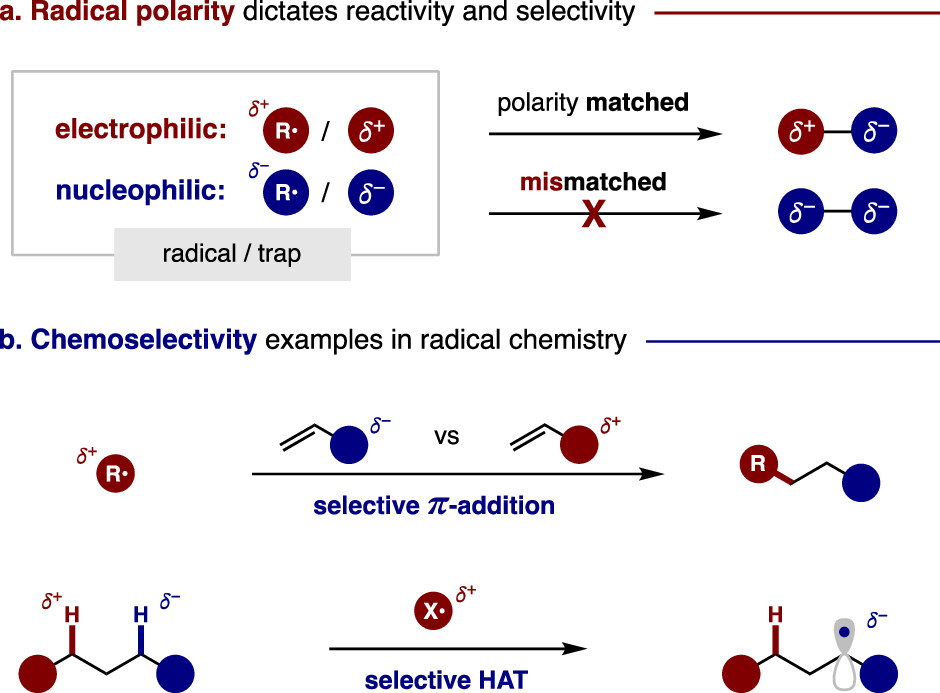
Notably, nucleophilic radicals are superior in both Giese π-additions to electron-deficient alkenes and Minisci aryl substitutions of pyridiniums. Conversely, electrophilic radicals are better suited for (anti-Markovnikov) Kharasch π-additions to electron-rich alkenes, as well as homolytic aromatic substitutions (SHAr) of electron-rich arenes. Similarly, homolytic substitution (SH2) reactions are strongly influenced by polarity. For example, HAT of hydridic C–H bonds are best mediated by an electrophilic radical (N•, O•), while abstraction of electrophiles by either group- (e.g., xanthate) or halogen atom transfer (XAT) are often facilitated by nucleophilic radicals (Sn•, Si•).
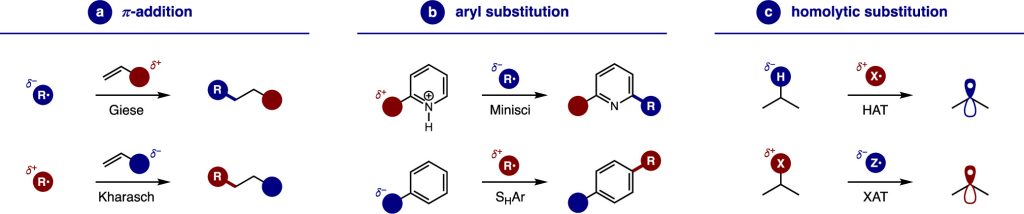
A clear understanding of radical nucleophilicity and electrophilicity enhances the unique potential of radical chemistry, guiding the design and development of novel, efficient, and highly selective radical reactions.
For examlple, Roberts and colleagues demonstrated site-selective hydrogen atom transfer (HAT) reactions in butyrolactone using either nucleophilic boron radicals or electrophilic tert-butoxy radicals.
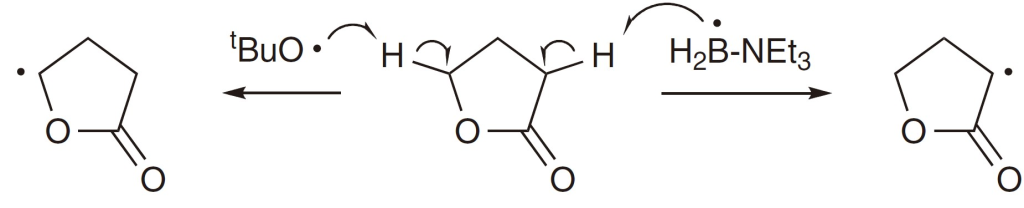
Though the bond dissociation energies (BDE) of the two C-H bonds involved were similar. However, the t-BuO· selectively abstracted hydrogen from the O α-position, whereas the Et3N-BH2· abstracted the hydrogen at the carbonyl group α-position.
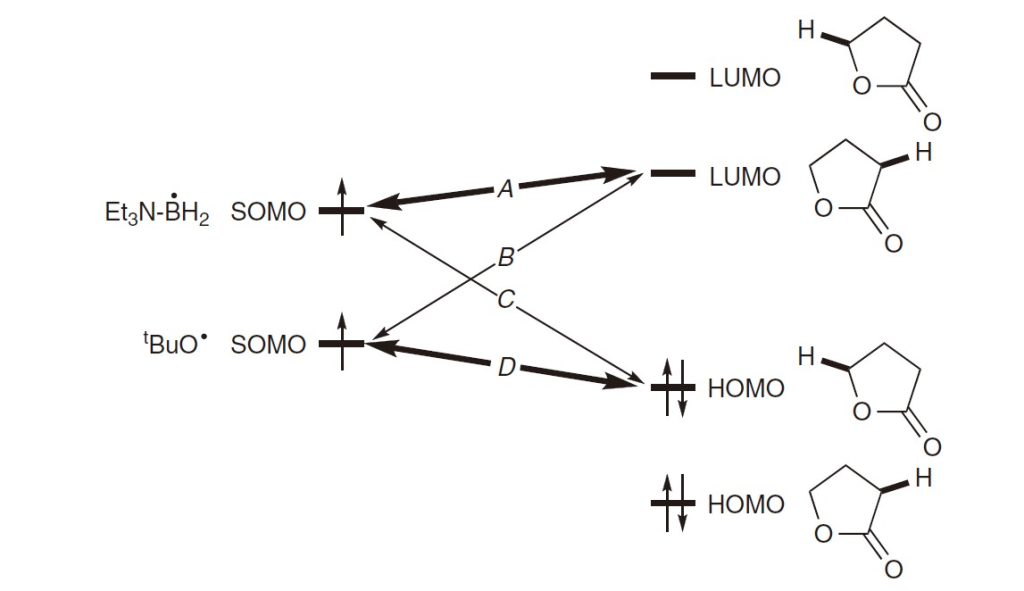
In this reaction, the key frontier orbitals involve the radical’s SOMO interacting with either the σ or σ* orbital of the C-H bond. Due to the high electronegativity of oxygen, the tert-butoxy radical (t-BuO·) has a relatively low-energy SOMO, which interacts strongly with high-energy orbitals adjacent to electron-donating (X) substituents (as in D).
In contrast, the triethylamine-borane radical (Et₃N-BH₂·) has a high-energy SOMO, making it more likely to interact with lower-energy σ* orbitals of C-H bonds (like A). Thus, for Et₃N-BH₂·, A is more reactive than C, while for t-BuO·, D is more reactive than B.
How to Evaluate Radical Polarity?
Radical polarity can be roughly evaluated using the following four methods.
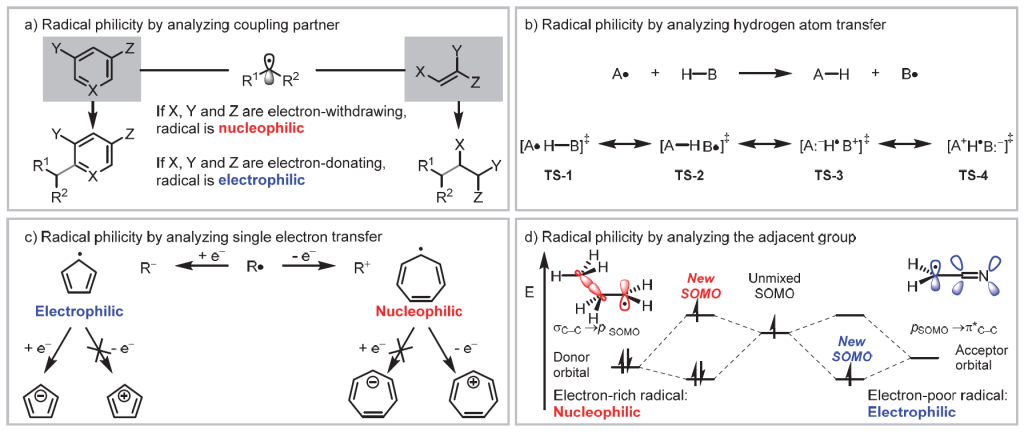
- a. Similar to differentiating between nucleophiles and electrophiles in polar chemistry, radicals that readily react with electron-deficient species are classified as nucleophilic radicals, while those reacting with electron-rich species are considered electrophilic. Radical reactions following this polarity-matching principle tend to be kinetically more favorable.
- b. Roberts proposed evaluating radical polarity based on the charge-separated structures in hydrogen atom transfer (HAT) reaction transition states. If the contribution of TS-3 outweighs that of TS-4, radical A is electrophilic, and radical B is nucleophilic. Conversely, if TS-4 dominates, radical A is nucleophilic, and B is electrophilic.
- c. Cheng Jinpei and colleagues determine radical polarity by examining the stability of the anions or cations formed when radicals gain or lose an electron. For example, the cyclopentadienyl radical easily forms an aromatic anion upon gaining an electron, classifying it as an electrophilic radical. In contrast, the cycloheptatrienyl radical forms a stable aromatic cation when losing an electron, making it a nucleophilic radical.
- d. Calculation methods.
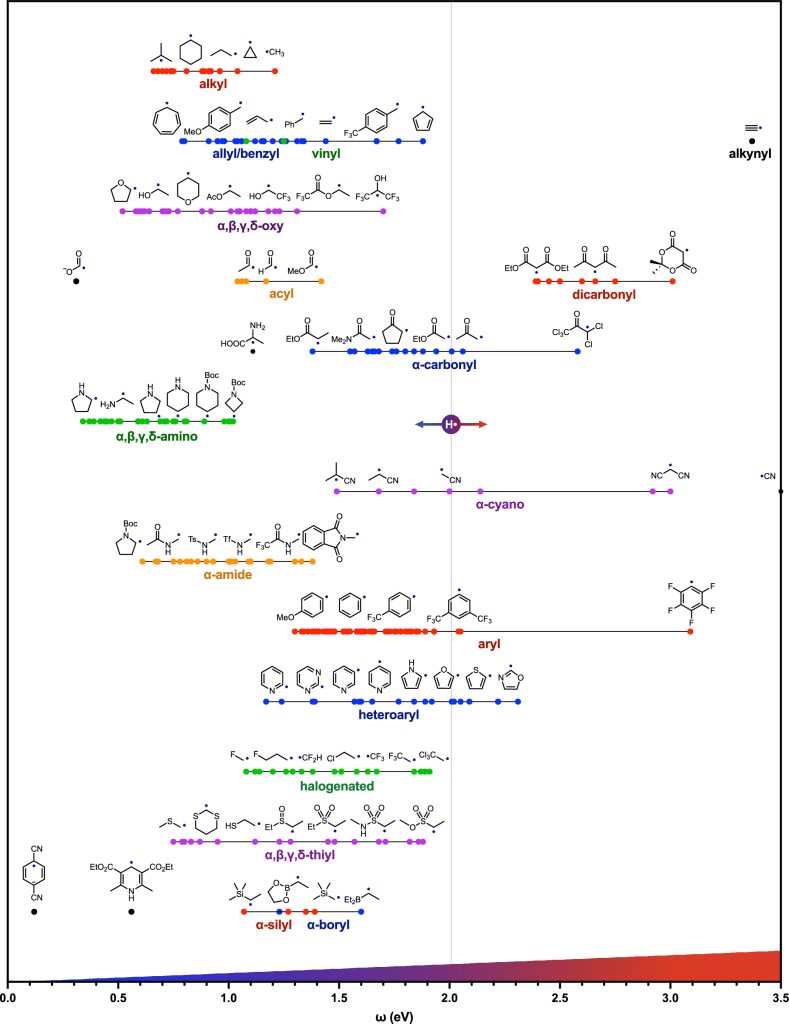
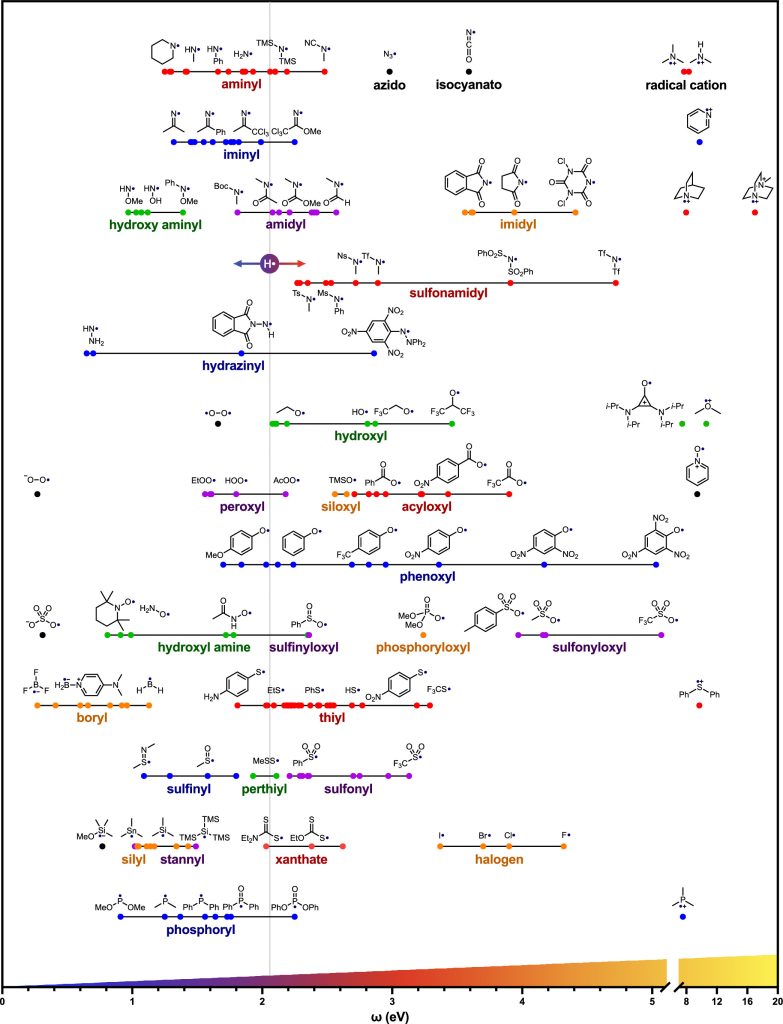
Recently, David A. Nagib and his collegues compiled a list of the most common radicals encountered in organic synthesis and calculated their electrophilicity (ω) at the B3LYP-D3/6-311 + G** level of theory. Here are some of their calculation results.
(Nice work! though I’m not skilled in computational chemistry, I still think it’s an excellent tool that offers valuable guidance to researchers. )
References
- Parsaee, F.; Senarathna, M. C.; Kannangara, P. B.; Alexander, S. N.; Arche, P. D. E.; Welin, E. R. Radical Philicity and Its Role in Selective Organic Transformations. Nat Rev Chem 2021, 5 (7), 486–499.
- Garwood, J. J. A.; Chen, A. D.; Nagib, D. A. Radical Polarity. J. Am. Chem. Soc. 2024, 146 (41), 28034–28059.
- Le, C.; Liang, Y.; Evans, R. W.; Li, X.; MacMillan, D. W. C. Selective Sp3 C–H Alkylation via Polarity-Match-Based Cross-Coupling. Nature 2017, 547 (7661), 79–83.