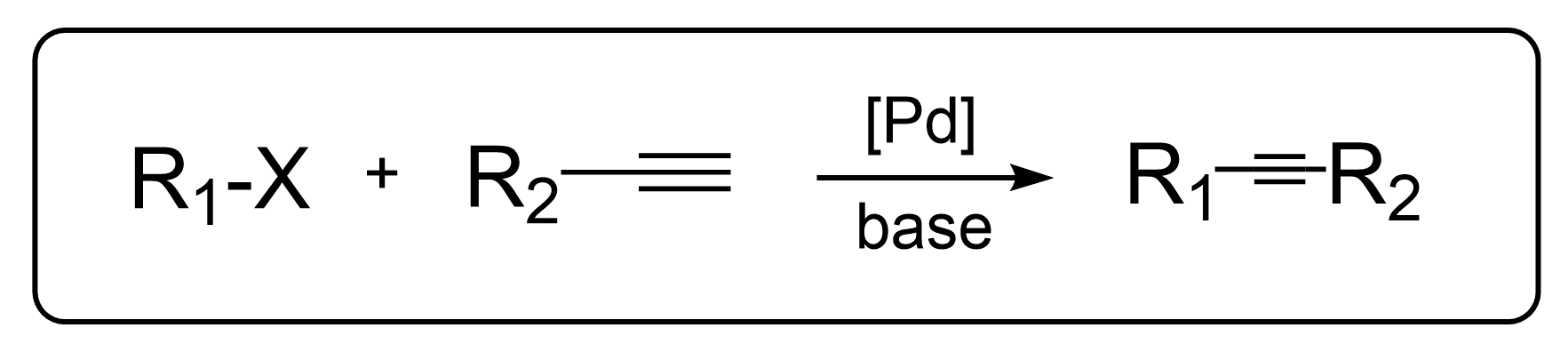
1. Intro
The Sonogashira cross-coupling reaction refers to the reaction between organic electrophilic reagents and terminal alkynes. It is one of the most important methods for forming carbon-carbon bonds and synthesizing various alkynes, particularly internal alkynes. This reaction has been widely applied in the synthesis of natural products, bioactive compounds, and in material science.
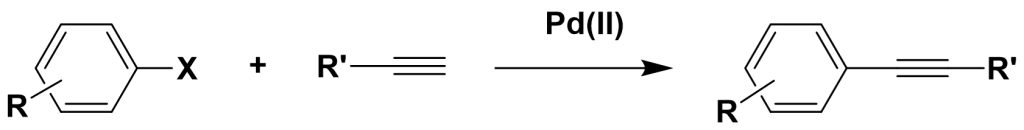
2. Limitations of Early Sonogashira Reactions
The commonly used catalytic system for the Sonogashira cross-coupling reaction consists of palladium(II), phosphine ligands, and copper iodide (CuI). The most frequently employed ligands in palladium-catalyzed coupling reactions are monodentate, sterically hindered, and electron-rich triarylphosphines.
- Limitation 1: In the presence of oxidants or air, copper salts (CuI) can form copper-alkyne complexes, which further lead to the self-coupling of terminal alkynes. This side reaction significantly reduces the yield of the target compound, especially when terminal alkynes are scarce.
- Limitation 2: The commonly used phosphine ligands are often sensitive to air and moisture, expensive, and toxic. The reaction conditions are harsh, and the ligands tend to decompose to form phosphates and other byproducts upon heating. Recovery and synthesis of these ligands are difficult, making the entire process costly. Additionally, the reaction must be conducted under an inert atmosphere, making industrial-scale applications challenging.
- Limitation 3: The catalytic systems for existing Sonogashira cross-coupling reactions have limitations regarding the substrates used. Typically, these systems work well with aryl iodides, bromides, and activated aryl chlorides. Thus, the development of an efficient Sonogashira cross-coupling catalytic system has become a major research goal for many scholars worldwide.
3. Recent Advances in Sonogashira Reaction
In recent years, significant progress has been made in the following areas of Sonogashira reaction research:
- Introduction of new ligands;
- Discovery of novel catalytic systems;
- Expansion of substrate variety;
- Broader application range.
3.1 Introduction of New Ligands
By introducing N-heterocyclic carbene (NHC) ligands into the original catalytic system (Pd(OAc)2, PPh3, and CuI) and replacing the solvent with DMF, higher yields have been achieved.
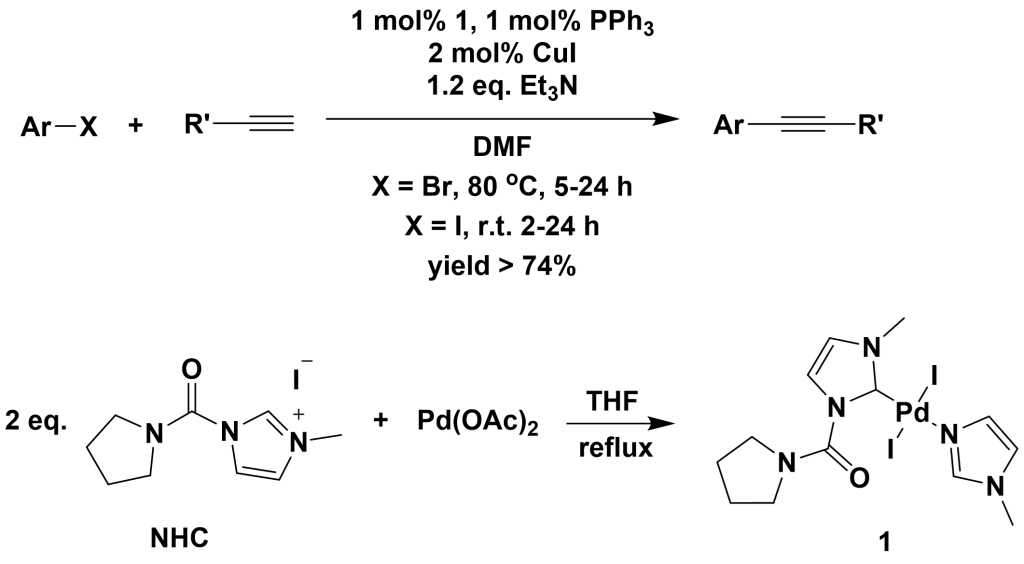
Additionally, Li et al. studied the use of tertiary amines as ligands in palladium-catalyzed Sonogashira cross-coupling reactions. They found that when DABCO was used as the ligand, various iodinated and more reactive brominated aromatic compounds could efficiently undergo Sonogashira cross-coupling with terminal alkynes. The amount of palladium acetate could be reduced to as low as 0.0001 mol%, achieving a catalytic efficiency as high as 720,000.
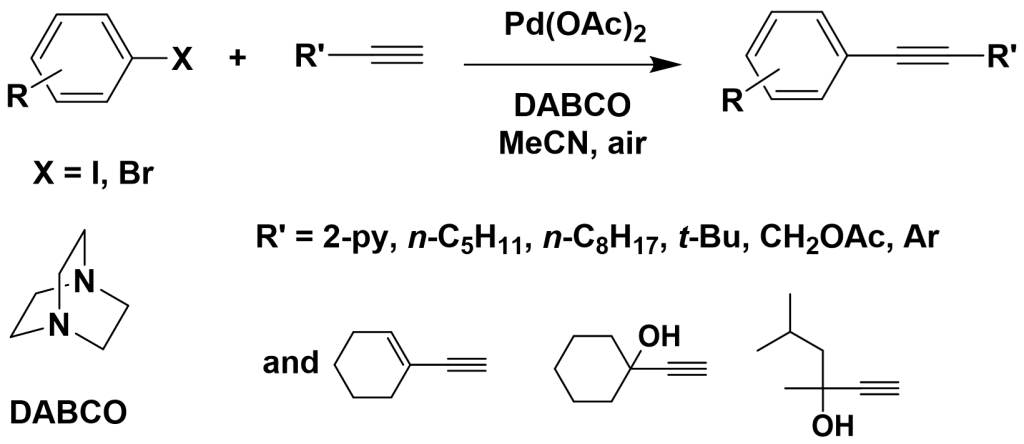
3.2 Discovery of Novel Catalytic Systems
3.2.1 Hydrogen and Inert Gas Environment Catalytic Systems
In the presence of oxidants or air, copper(I) salts (CuI) can form copper-alkyne complexes, leading to the self-coupling of terminal alkynes. Elangovan et al. demonstrated that conducting the reaction under a hydrogen, nitrogen, or argon atmosphere can reduce the side reaction of terminal alkyne self-coupling to 2%, allowing for higher yields of the Sonogashira cross-coupling products.
3.2.2 Copper-Free Palladium Catalytic Systems
Copper-free palladium catalytic systems are composed of palladium catalysts and bases, with common palladium catalysts including Pd(OAc)2, Pd2(dba)3, PdCl2(dppf), PdCl2(PPh3)2, PdCl2(PCy)2, PdCl2, and PdCl2(CH3CN)2. Commonly used bases include Cs2CO3, Bu4NOAc, TBAF, Pyrrolidine, TEA, and DBACO. The advantage of copper-free palladium catalytic systems is their insensitivity to air and the formation of only trace amounts of terminal alkyne self-coupling products.
3.3 Expansion of Substrate Variety
The variety of substrates has expanded to include not only aryl halides but also aryl sulfonates, vinyl halides, allyl halides, aliphatic halides, and acyl chlorides.
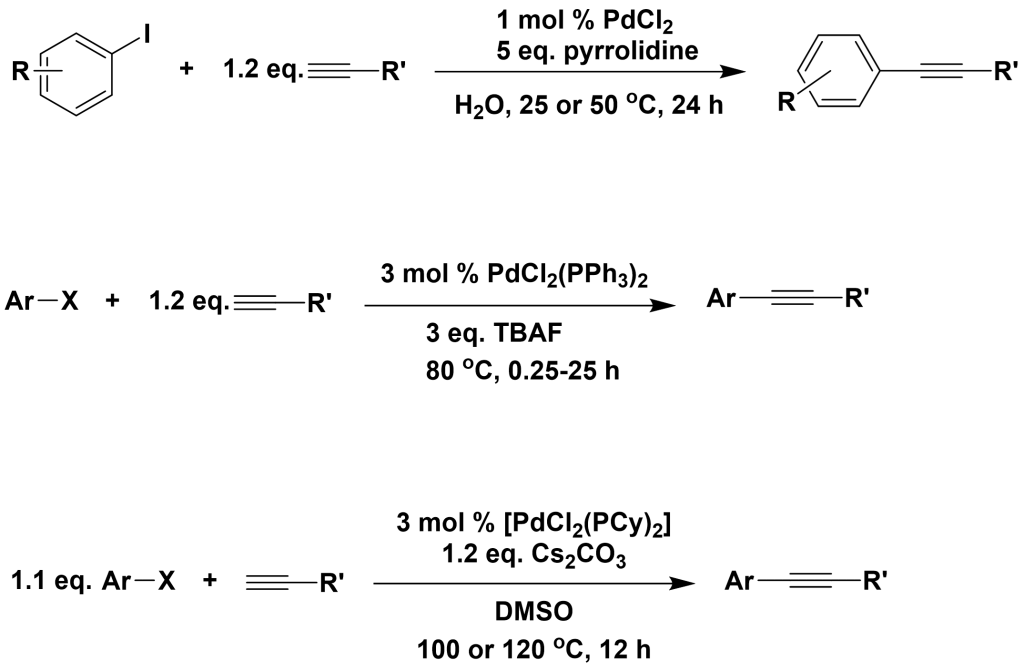
J. Org. Chem., 2006, 71, 379-381
J. Org. Chem., 2006, 71, 2535-2537
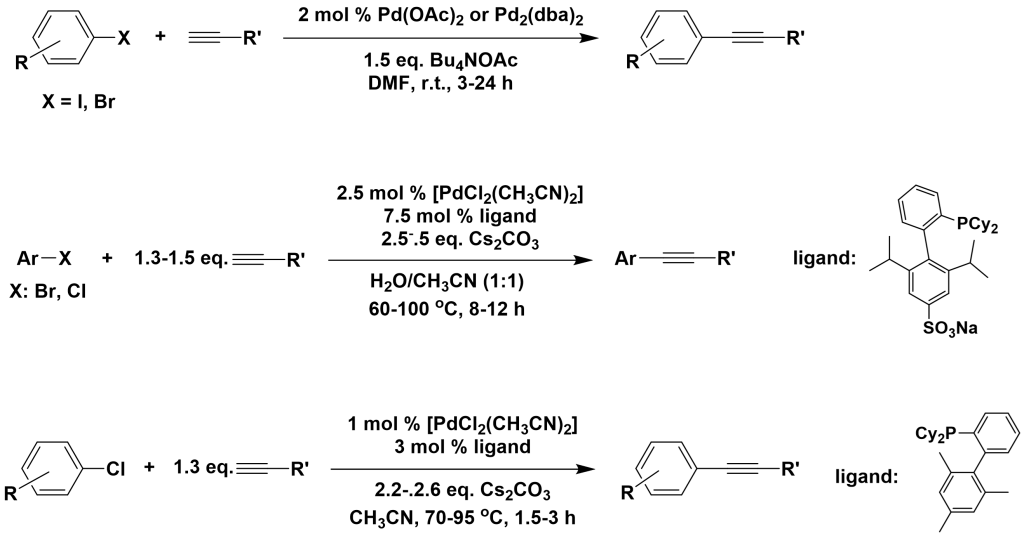
Angew. Chem., 2005, 117, 6329
Angew. Chem., 2003, 115, 6175
3.3.1 Coupling of Aryl Sulfonates with Terminal Alkynes
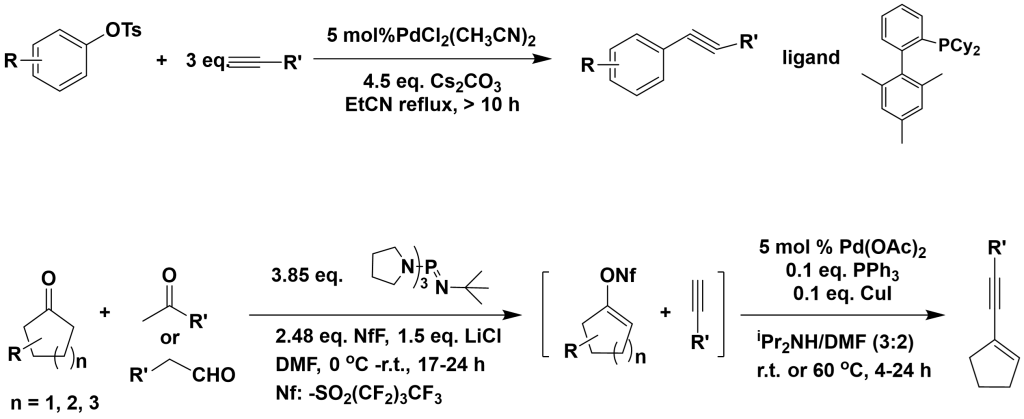
Angew. Chem. Int. Ed., 2006, 45, 4019
3.3.2 Coupling of Vinyl Halides with Terminal Alkynes
Org. Lett., 2004, 6, 1441
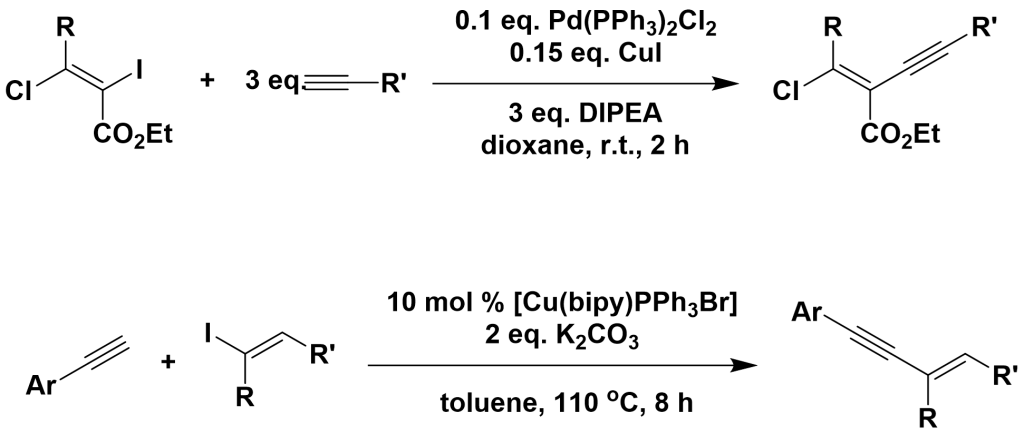
3.3.3 Coupling of Aliphatic Halides with Terminal Alkynes
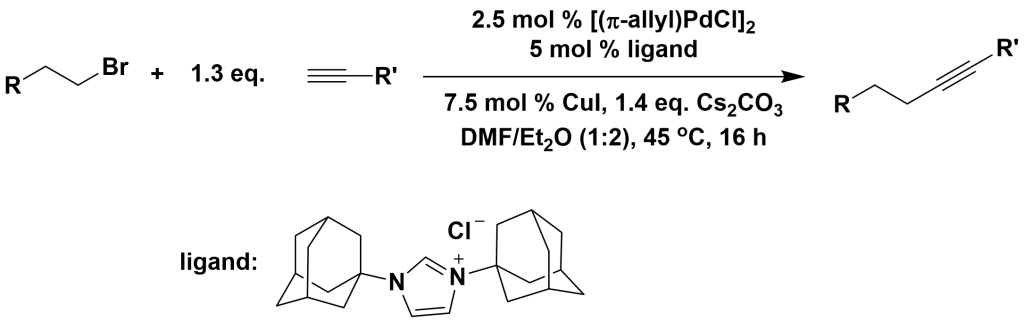
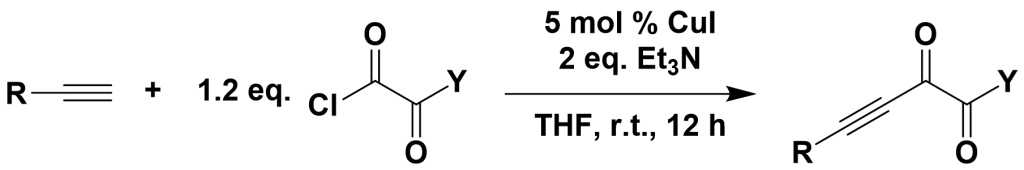
3.3.4 Coupling of Acyl Chlorides with Terminal Alkynes
3.4 Expanded Applications
Research on the Sonogashira cross-coupling reaction has not only delved deeply into theoretical studies but has also led to the development of numerous practical applications.
3.4.1 Synthesis of Flavonoids
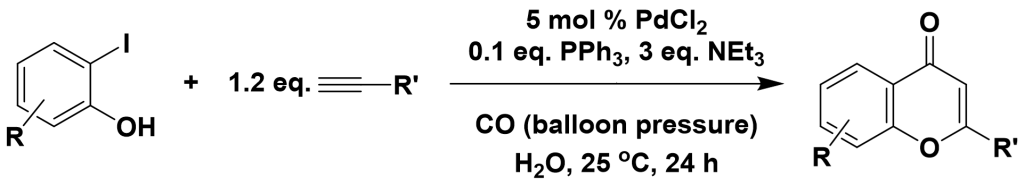
3.4.2 Synthesis of α,β-alkynyl ketones
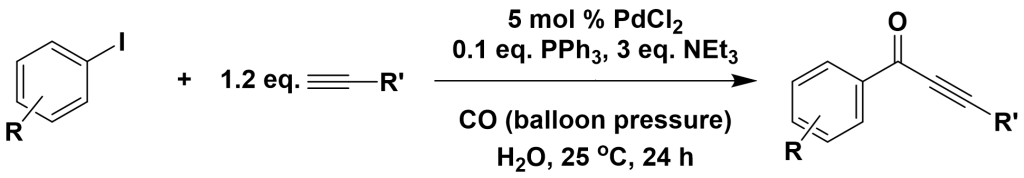
Org. Lett., 2003, 5, 3057-3060
3.4.3 Synthesis of propargylamines
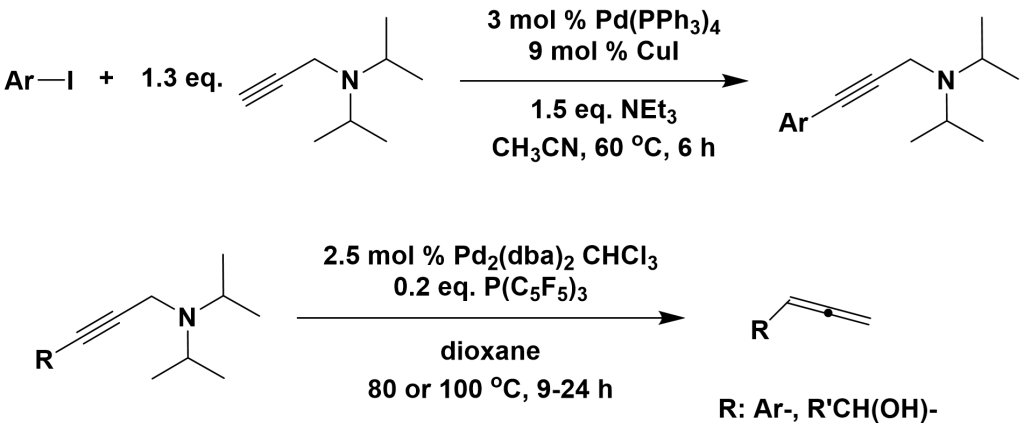
J. Am. Chem. Soc. 2004, 126, 8638
The synthesis of propargylamine derivatives via hydrogen transfer reactions under palladium catalysis readily leads to the formation of propargyl compounds, making it a potential plasma source for propargyl compounds. This characteristic allows its application in supramolecular chemistry.