Intro
As one of the most intuitive detection methods, fluorescent probes are now widely applied across various analytical settings. To date, researchers have proposed numerous photochemical models to theoretically explain the mechanisms underlying fluorescent probes, including ICT, TICT, FRET, PET, and ESIPT. Understanding these photochemical processes helps facilitate rational design and a deeper comprehension of fluorescence-based technologies. This article aims to provide a concise analysis of these well-known photochemical concepts in fluorescent probe technology. Notably, many of these concepts also find broad applications in fields such as photocatalysis, photodynamic therapy, and optoelectronic materials.
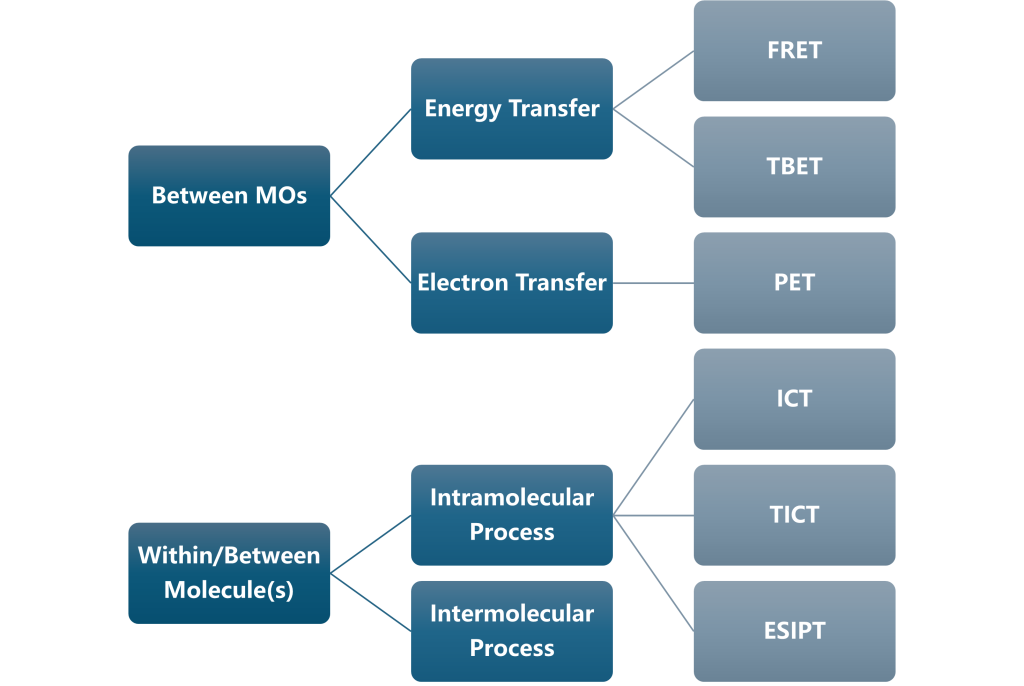
In general, the deactivation pathways of excited-state molecules in photochemical processes can be categorized as follows: radiative transitions (such as fluorescence and phosphorescence), non-radiative transitions (such as internal conversion and intersystem crossing), chemical reactions, energy transfer, and electron transfer.
Key concepts related to energy transfer include fluorescence resonance energy transfer (FRET) and through-bond energy transfer (TBET). In the context of electron transfer, photoinduced electron transfer (PET) is a classic concept. Additionally, models such as intramolecular charge transfer (ICT), twisted intramolecular charge transfer (TICT), and excited-state intramolecular proton transfer (ESIPT) are frequently mentioned in the literature.
Let’s start!
Fluorescence Resonance Energy Transfer (FRET)
Fluorescence Resonance Energy Transfer (FRET) occurs when a donor fluorophore (Fluorophore A) absorbs photons of a specific frequency and transitions to an excited electronic state. Before the donor returns to its ground state, it non-radiatively transfers energy to a nearby acceptor fluorophore (Fluorophore B) through dipole-dipole interactions, exciting the acceptor. The acceptor then returns to its ground state via a radiative transition, emitting fluorescence. This energy transfer, facilitated by dipole-dipole coupling, is a non-radiative process. The theory considers the excited donor molecule as an oscillating dipole that can exchange energy with another dipole oscillating at the same frequency, hence the term “fluorescence resonance energy transfer.”
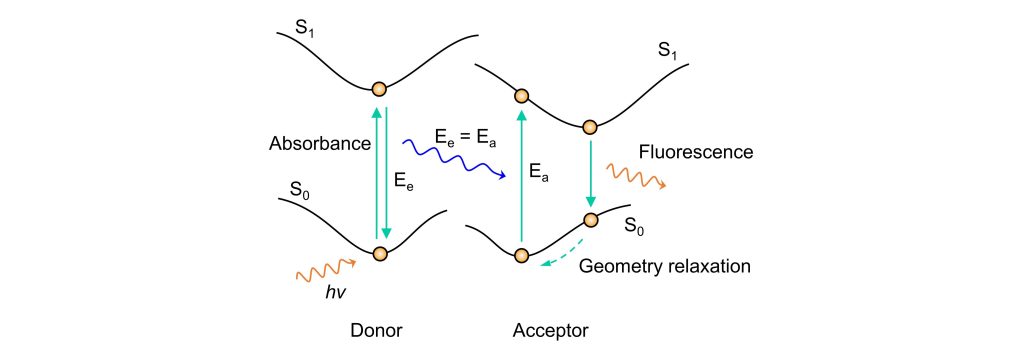
From a potential energy surface perspective, as shown in the diagram, the donor’s electrons are excited to a higher state by photon absorption. During the return to the ground state, the energy released via dipole-dipole interaction matches the energy needed to excite the acceptor, transferring this energy to the acceptor. This excites the acceptor’s electrons from the ground to an excited state. After undergoing internal conversion and vibrational relaxation, the acceptor returns to its ground state by emitting fluorescence.
For FRET to occur, four main conditions must be met:
- The donor and acceptor must be physically close to each other.
- The emission spectrum of the donor must overlap with the absorption spectrum of the acceptor.
- The excitation spectra of the donor and acceptor must be sufficiently separated.
- The dipole moments of the donor and acceptor must be aligned in a specific orientation.
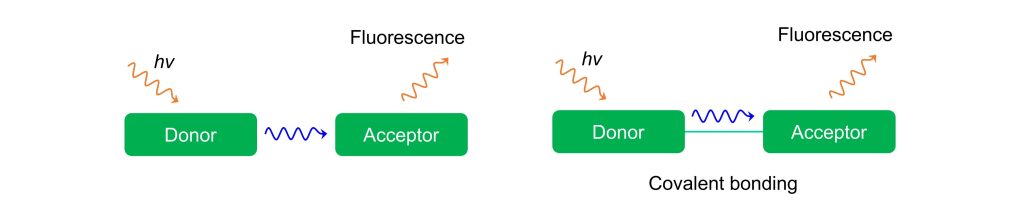
Based on this theoretical framework, FRET can occur as long as two fluorophores meet these four conditions, regardless of whether they are covalently linked. Literature research confirms this understanding—FRET can take place both between two separate molecules and within a single molecule.
Through-Bond Energy Transfer (TBET)
Through-Bond Energy Transfer (TBET) differs from FRET in its mode of energy transfer. In TBET systems, the donor transfers energy to the acceptor through a rigid, electronically conjugated structure, such as a phenyl group or a triple bond. Despite this difference, the fundamental mechanism of TBET is similar to that of FRET, as both involve energy transfer. Therefore, TBET can be considered a special case of FRET.
In TBET, the conjugated structure linking the donor and acceptor reduces the strict requirements seen in FRET’s dipole-dipole interaction-based mechanism. This structural feature leads to higher energy transfer efficiency compared to FRET. However, designing TBET-based molecules remains challenging, as the system can still be influenced by FRET mechanisms.
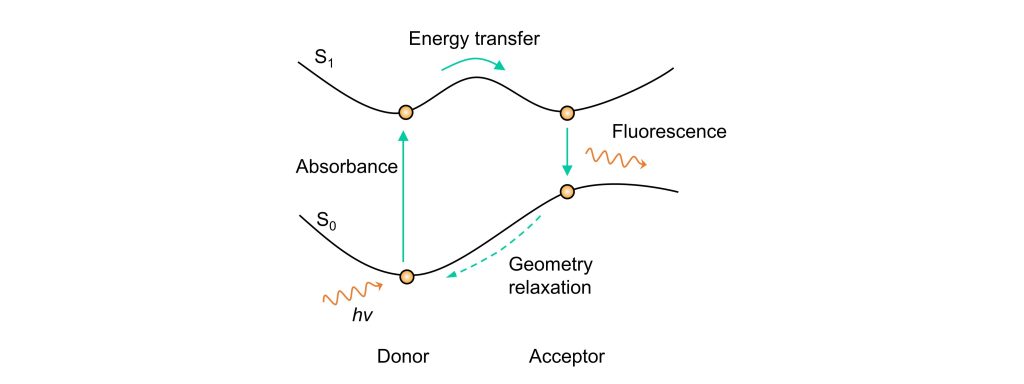
From a potential energy surface perspective, as shown in the diagram, the covalent connection between the donor and acceptor allows their potential energy surfaces to merge. Unlike FRET, where energy must be released and then absorbed, TBET facilitates a direct transfer of energy to the acceptor without this intermediate step.
Photoinduced Electron Transfer (PET)
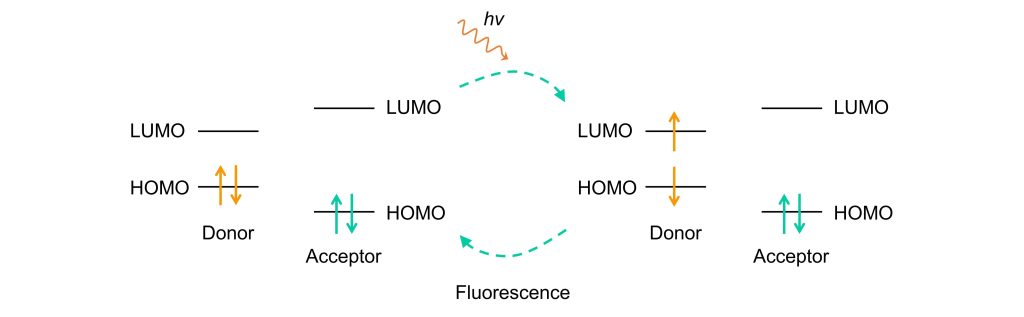
In photoinduced electron transfer (PET) fluorescent probes, an electron transfer occurs between the donor (fluorophore) and the acceptor unit, which strongly quenches fluorescence. Typically, an electron is transferred from the donor to the excited fluorophore. As a result, before binding to a target molecule, the probe emits no or very weak fluorescence. Once the acceptor binds to the target, the PET process is inhibited or completely blocked, allowing the fluorophore to emit fluorescence.
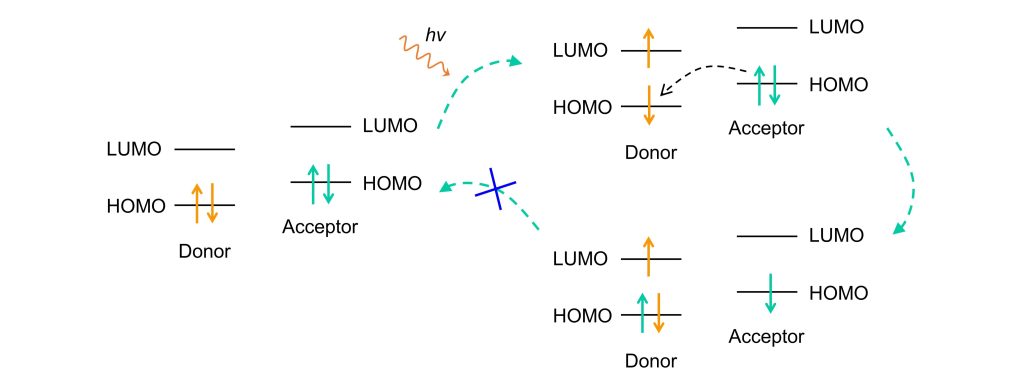
PET-based fluorescent probes are generally classified into two types: Donor-PET and Acceptor-PET, depending on the quenching mechanism.
1. Donor-PET:
In Donor-PET systems:
- The donor has the lowest HOMO energy level, and its LUMO energy lies between the acceptor’s HOMO and LUMO levels.
- Upon excitation, the electron in the donor is promoted to the LUMO. Since the acceptor’s HOMO energy is lower than the donor’s LUMO, an electron from the acceptor’s HOMO readily transfers to the donor’s HOMO.
- This electron transfer prevents the excited electron in the donor’s LUMO from returning to its HOMO, resulting in fluorescence quenching.
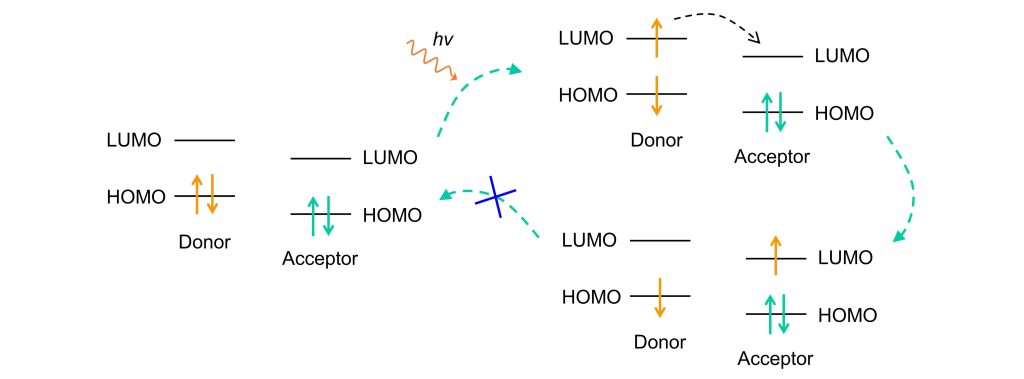
2. Acceptor-PET:
In Acceptor-PET systems:
- The acceptor has the lowest HOMO energy level, and its LUMO energy lies between the donor’s HOMO and LUMO levels.
- When the donor is excited, the electron in the donor’s LUMO is more likely to transfer to the acceptor’s LUMO due to the small energy gap.
- As a result, the electron does not return to the donor’s HOMO, leading to fluorescence quenching.
Fluorescence can only be restored when the donor’s HOMO and LUMO energy levels are positioned between the acceptor’s HOMO and LUMO. In this case, the excited electron in the donor’s LUMO returns to the HOMO via radiative transition, emitting fluorescence.
In PET fluorescent probes, the interaction or binding of the acceptor (recognition group) with the target molecule alters the acceptor’s HOMO and LUMO energy levels. This change effectively suppresses the PET process, resulting in fluorescence restoration and enabling target detection.
Intramolecular Charge Transfer (ICT)
Intramolecular Charge Transfer (ICT), also known as Photo-induced Charge Transfer (PCT), is a crucial strategy for designing ratiometric probes. These probes typically consist of an electron-donating group (donor), an electron-withdrawing group (acceptor), and a π-conjugated bridge. Within the molecule, an electron transfer process occurs from the donor through the π-bridge to the acceptor, known as ICT. The fluorescence induced by this effect is referred to as ICT emission.
For sensing probes, either the donor or acceptor group is often modified with a recognition moiety. This modification initially blocks the ICT emission. Only when the recognition group interacts or reacts with the target molecule does it alter the electron-donating or withdrawing properties, enabling ICT emission and facilitating the detection of the target.
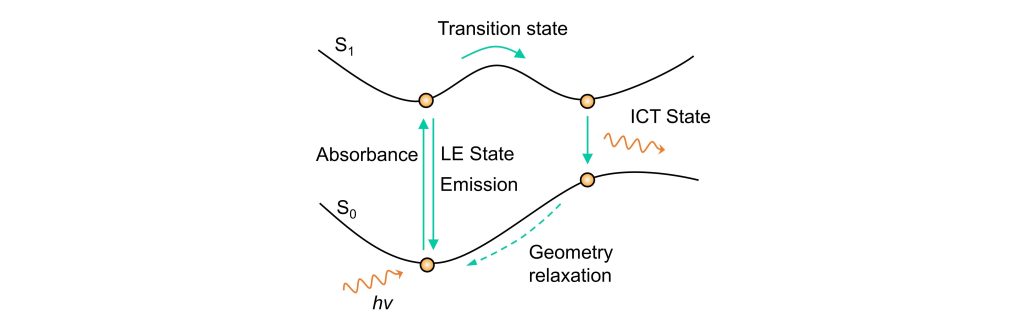
From a potential energy surface perspective, as shown in the diagram, before interacting with the target, the molecule returns to its ground state via a localized charge transfer (LE transition) upon excitation. After interacting with the target, the electron-donating or withdrawing abilities of the groups change, allowing the excited electron to overcome a transition state (TS) and reach the ICT state, leading to ICT emission.
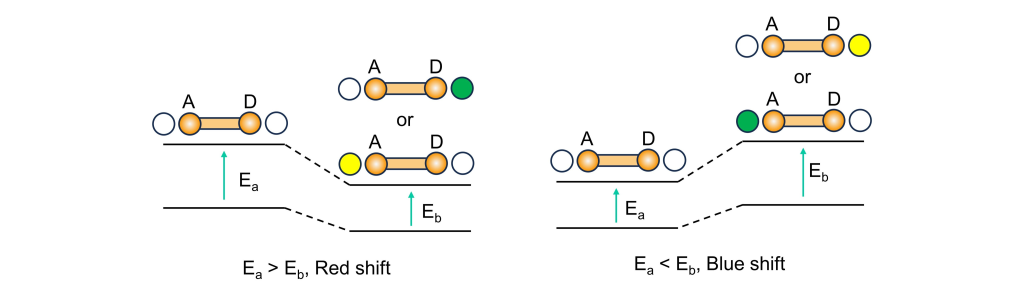
For ratiometric probes, this wavelength shift is illustrated clearly in the diagram. When the electron-donating ability of the donor decreases or the electron-withdrawing ability of the acceptor increases, the energy gap narrows, resulting in a red shift in the emission wavelength—a common strategy in ICT probes. Conversely, when the donor’s electron-donating ability increases or the acceptor’s electron-withdrawing ability decreases, the energy gap widens, causing a blue shift in the emission wavelength.
Twisted Intramolecular Charge Transfer (TICT)
Twisted Intramolecular Charge Transfer (TICT) differs structurally from the planar conjugated system of ICT. In TICT systems, the donor and acceptor are connected via a single bond, creating a certain dihedral angle—sometimes approaching orthogonality. Fundamentally, TICT still relies on charge delocalization, making it a specific case of ICT.
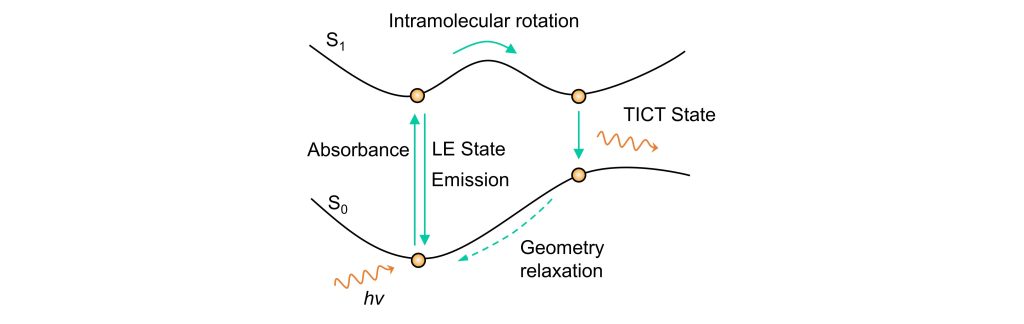
From a potential energy surface perspective, as shown in the diagram, the molecule is first excited to its excited state. Through structural twisting, it transitions into the TICT state. Because the excited-state structure undergoes significant conformational changes, a large portion of the energy is dissipated through non-radiative transitions as the molecule returns to its deformed ground state.
In ICT systems, electron transfer occurs within a single planar structure. In TICT systems, however, electron transfer takes place between two planes at a specific angle, sometimes even perpendicular to each other.
Excited-State Intramolecular Proton Transfer (ESIPT)
Excited-State Intramolecular Proton Transfer (ESIPT) occurs when a probe molecule, upon light excitation, undergoes electronic transition from the ground state to the excited state. During this process, a hydrogen proton within the molecule transfers via intramolecular hydrogen bonding to a neighboring heteroatom, such as N, O, or S. This induces an enol-to-keto tautomerization between the ground and excited states, forming the respective tautomers. The ESIPT process is fully reversible, operating as a cyclic transition.
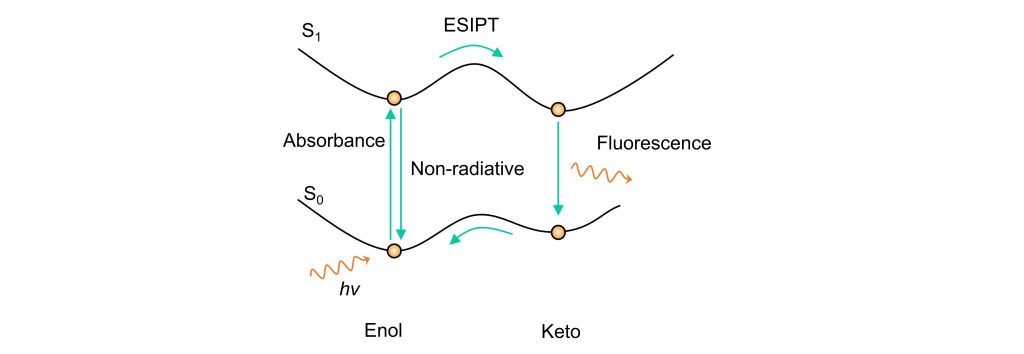
From a potential energy surface perspective, as shown in the diagram, the molecule in its enol form is excited to the excited state. In the excited state, the molecule undergoes a transformation from enol to keto form. The molecule then returns to the ground state via radiative transition (fluorescence), and in the ground state, it reverts from keto to enol form, recovering its initial structure.
Question:
What’s the difference between Charge Transfer (CT) and Electron Transfer (ET)?
