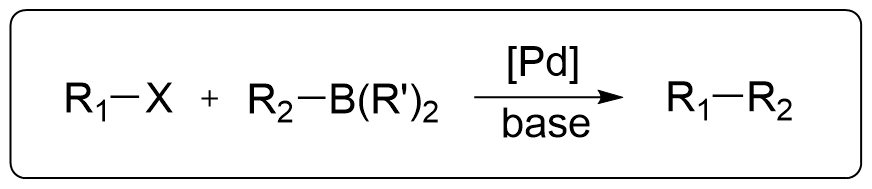
Intro
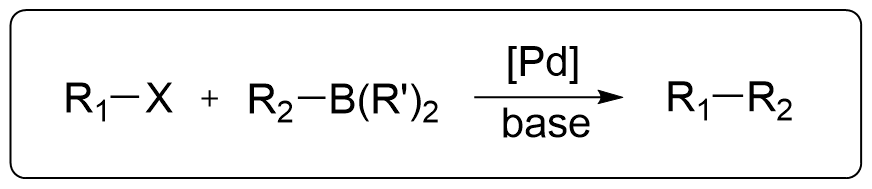
Also known as the Suzuki Cross-Coupling Reaction or Suzuki Reaction. It is one of the five major Pd-catalyzed cross-coupling reactions. This reaction involves the coupling of organoboranes with halides under the catalysis of Pd and base, and it is among the most widely used coupling reactions today. Prof. Akira Suzuki was awarded the Nobel Prize in Chemistry in 2010 for this discovery.
Reaction characteristics:
- Not sensitive to water.
- Excellent functional group tolerance: -COOC2H5, -OCH3, -CN, -NO2, and -F can undergo the reaction without being affected.
- Some heterocyclic boronic acids and Ar-Cl, especially those with significant steric hindrance, can be hard to react.
- Less toxic and safer for the environment than organotin and organozinc compounds.
- Easy to remove the inorganic by-products from the reaction mixture.
- Relatively cheap and easily prepared reagents.
Mechanism
The mechanism of the Suzuki-Miyaura coupling reaction primarily involves three classic steps:
- Oxidative addition
- Transmetalation
- Reductive elimination
For the Oxidative Addition, the lower the BDE of the C–X bond and the stronger the polarization (the more electron-deficient the aromatic ring), the more favorable the oxidative addition to Pd becomes. So The general reactivity order of different X is: I–>OTf–>Br–>>Cl–
For Reductive Elimination, the general reactivity order of different R1-R2 is: aryl–aryl > alkyl–aryl > n-propyl–n-propyl > ethyl–ethyl > methyl–methyl.
Common Side Reaction
R1-X Dehalogenation:
In the mechanism, the higher the concentration of R1PdXLn formed through oxidative addition, the greater the possibility of dehalogenation in subsequent steps. Therefore, to control the concentration of R1PdXLn, the reactivity of the halide, which serves as the oxidative addition substrate, should not be too high. The higher the reactivity of the halide, the more electron-deficient the aromatic ring, and the stronger the base used, the more dehalogenation becomes. If highly reactive halides tend to undergo dehalogenation, a weaker base can be used as a substitute.
R2-B(OR)2 Deboronation:
Arylboronate Ester self-coupling:
Electron-rich substrates are more possible to this type of side-reaction. The Suzuki reaction must be conducted under an inert atmosphere, one of the reasons is that the presence of oxygen can accelerate the self-coupling of arylboronate esters.
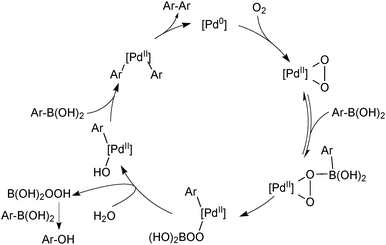
Arylboronic acid Oxidation
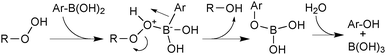
Aerobically-generated peroxide-type oxidants can readily form in many ethereal solvents, and boronic acids are highly susceptible towards oxidation by these species under coupling conditions. Arylboronic acids form phenols following a 1,2-migration of the aryl moiety to an electrophilic oxygen atom.
Application
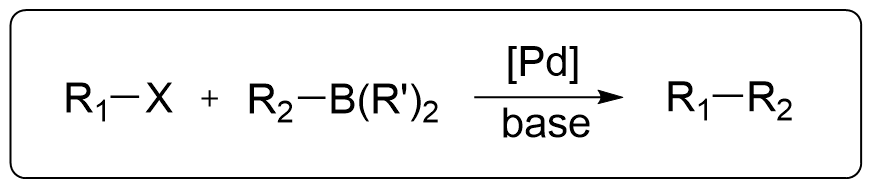
Classical reaction conditions:
- R1 = aryl, alkenyl, alkyl
- X = I, Br, Cl, OTf, OPO(OR)2
- R2 = aryl, alkenyl, alkyl, allyl, alkynyl
- R = alkyl, OH, O-alkyl
- [Pd] = Pd(PPh3)4, Pd(PPh3)2Cl2, Pd(dppf)Cl2,
- base = Na₂CO3, Ba(OH)2, K2COз, TIOH, KF, CS2CO3, K3PO4
- solvent: Toluene/H2O, THF/H2O, Dioxane/H2O, ACN/H2O
- N2 protected, heat
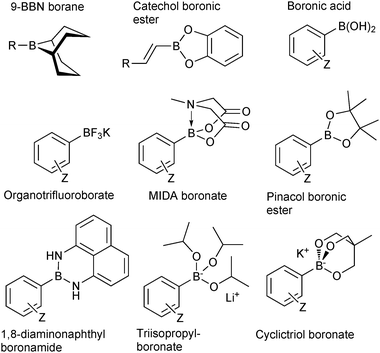
Common Boron reagent
Pd(0) or Pd(II) ?
Pd(PPh3)4 and Pd(PPh3)Cl2 have been used as catalyst of choice for a long time. Usually, Pd(0) catalysts are highly active, these compounds are typically air- and moisture-sensitive. The class of L2PdX2 catalysts is often stable toward air, moisture, and temperature.
Some Examples
Example 1
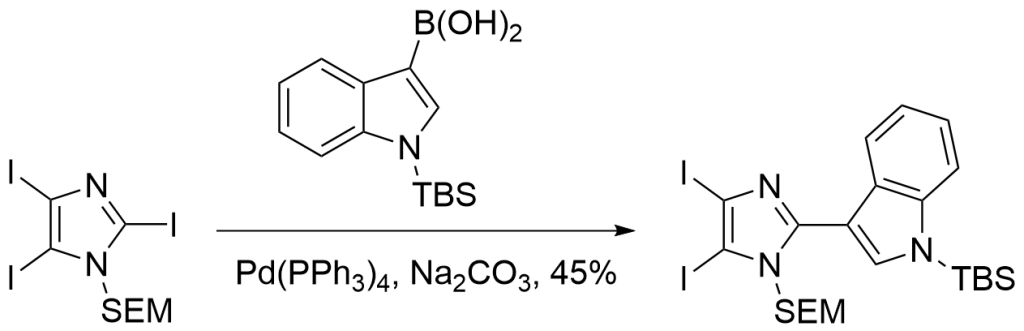
Example 2
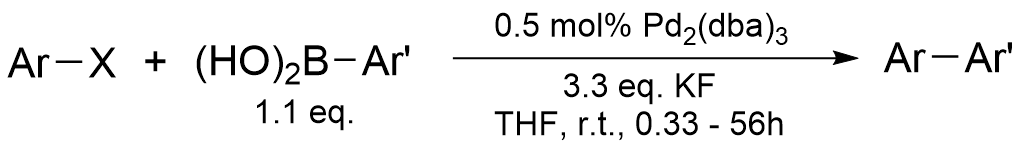
Example 3
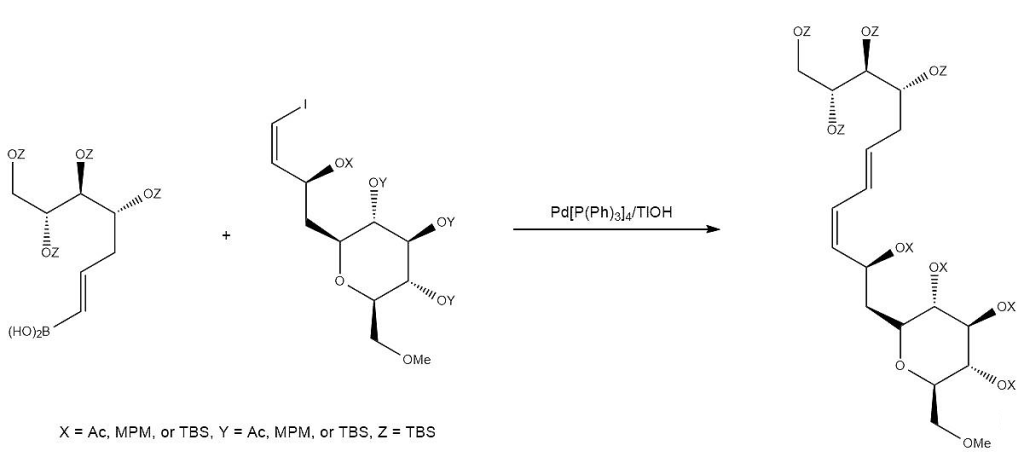
E. M. Suh, Y. Kishi, J. Am. Chem. Soc., 1994, 116, 11205–11206.
References
- N. Miyaura, K. Yamada, A. Suzuki, Tetrahedron Lett., 1979, 36, 3437–3440.
- J. H. Tidwell, A. J. Peat, S. L. Buchwald, J. Org. Chem., 1994, 59, 7164–7168.
- N. Miyaura, A. Suzuki, Chem. Rev., 1995, 95, 2457–2483.
- I. Kawasaki, H. Katsuma, Y. Nakayama, M. Yamashita, S. Ohta, Heterocycles, 1998, 48, 1887–1901.
- I. Kawasaki, M. Yamashita, S. Ohta, Chem. Pharm. Bull., 1996, 44, 1831–1839.
- G. A. Molander, F. Dehmel, J. Am. Chem. Soc., 2004, 126, 10313–10318.
- Kawasaki, I.; Kaisuma, H.; Nakayama, Y.; Yamashita, M.; Ohta, S. Heterocycles 1998, 48, 1887-1901.
- F. Littke; C. Dai, G. C. Fu; J. Am. Chem. Soc., 2000, 122, 4020–4028.
- E. M. Suh, Y. Kishi, J. Am. Chem. Soc., 1994, 116, 11205–11206.
Comments