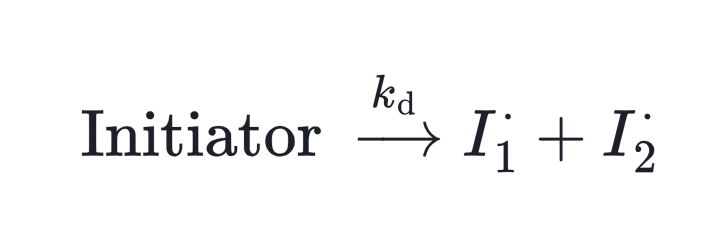Since August, I’ve been in Prof. Zhong’s group to work on radical polymerization research. It has been both exciting and rewarding, as I’ve already learned a great deal in just a few months. So I’m gonna summarize some of the fundamental concepts in polymer chemistry—starting with the basics of radical polymerization.
1 Introduction
Radical polymerization plays a central role both scientifically and industrially. Understanding its kinetics is essential for designing efficient routes to a wide range of polymer materials.
Here’re the basic kinetic steps of radical polymerization. In this post, I will try to give a clear overview of them and how these influence the process and products.
2 Initiation
Almost all radical polymerization starts with a free radical, which are generated by an initiator in the initiation step. This process is typically described by two parameters: the initiation rate coefficient (kᵢ) and the initiator efficiency (f).
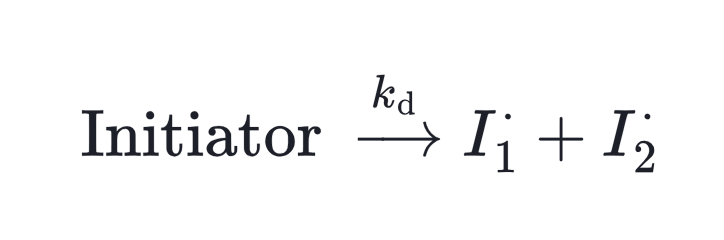
And most initiators fall into two categories:
- Thermal initiators, which form radicals when heated.
- Photoinitiators, which decompose when exposed to visible or UV light.
In industry area, thermal initiators dominate due to practicality. Meanwhile in kinetic studies photoinitiators are often preferred because irradiation can be precisely controlled, giving a sharp and clear starting point for the reaction.
Regardless of type, initiator decomposition acts as a first-order rate reaction.
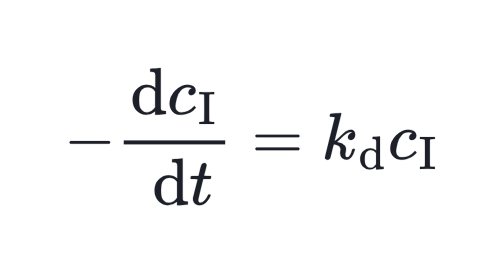
However, what really matters for polymerization is not just the initiator concentration but the number of primary radicals generated. This effective rate of radical formation (Rd) is expressed as:
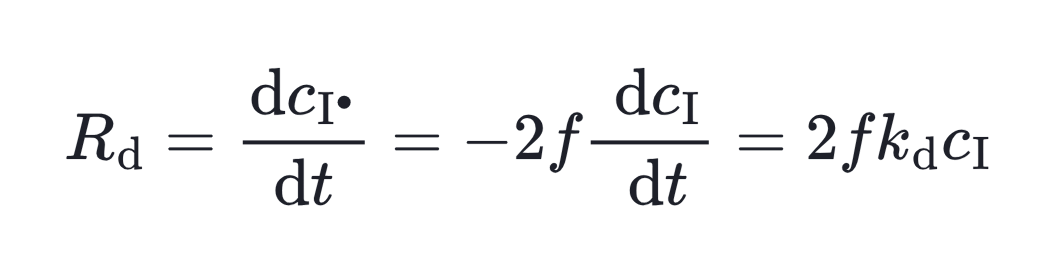
Here, kd is the decomposition rate constant and f is the initiator efficiency. Integrate this equation then we get the relation between initiator concentration and time.
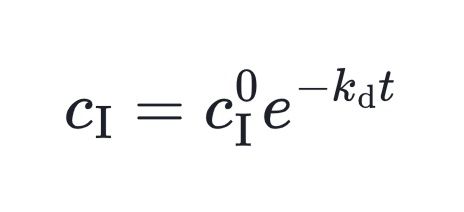
Importantly, initiator decay alone doesn’t guarantee new polymer chains. The newly formed radicals (I₁• and I₂•) must escape their initial “solvent cage” and react with monomers. The efficiency factor f accounts for this, reflecting how many radicals successfully initiate chains instead of recombining or being quenched.
f usually ranges between 0.5 and 0.8, strongly influenced by viscosity, because diffusion and escape from the solvent cage are key limiting factors.
2.1 Thermal Initiation
Thermal initiators are generally divided into two major classes: azo-type and peroxy-type.
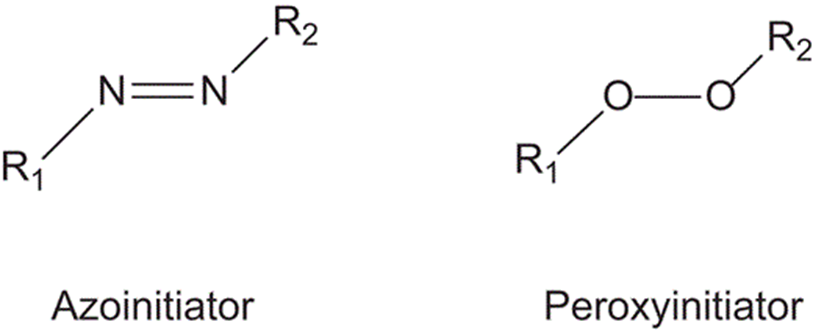
Upon heating, these initiators undergo first-order decomposition, which can be described by a characteristic half-life (t₁/₂).
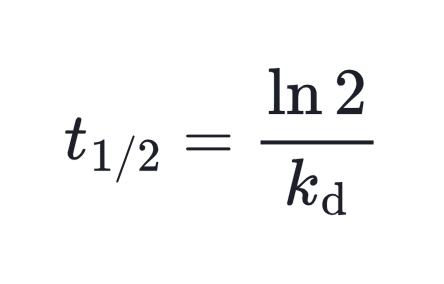
In practice, the choice of initiator often depends on its decomposition rate. A common benchmark is the temperature at which the initiator has a half-life of 10 hours. For typical thermal initiators, this temperature lies between 20 and 120 °C.
However, decomposition rate alone does not dictate the best choice. Other factors—such as potential side reactions with monomers or solvents, or whether the initiator can also function as a chain transfer agent (CTA) —may significantly influence its practical performance.
2.2 Photoinitiation
In research area, photoinitiators are widely used because they offer a unique advantage: the initiation process can be turned on and off with light. By simply adjusting the irradiation, one can precisely define when polymerization starts and stops. Another key difference from thermal initiators is that photoinitiators show little temperature dependence; instead, their decomposition rate strongly depends on the intensity of UV or visible light.
A good photoinitiator should ideally satisfy three conditions:
- Absorb strongly at a wavelength where neither the monomer nor solvent absorb.
- Generate radicals with high efficiency.
- Produce only a single radical species.
Photoinitiators are generally divided into two categories:
- Type I initiators: undergo unimolecular bond cleavage upon irradiation, directly generating radicals (similar to thermal initiators).
- Type II initiators: first enter an excited state after irradiation, then interact with a co-initiator (often via hydrogen abstraction) to produce radicals. Most visible-light initiators belong to this second type.
One of the most common model for Type I initiators is acetophenone group.
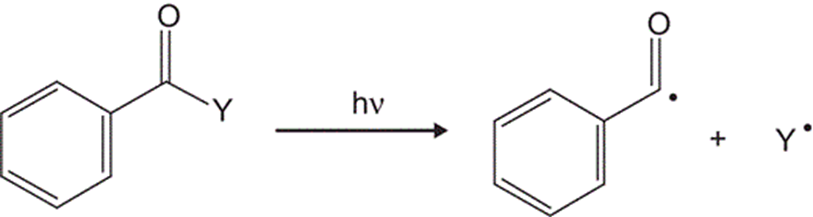
When a photoinitiator is lighted, it transitions from the ground state to an excited singlet state (S₁), as shown in a Jablonski diagram (Figure 1.1). From there, several relaxation pathways are possible. Only some of these lead to radical generation, which means the overall quantum yield (Φ) is usually less than one.
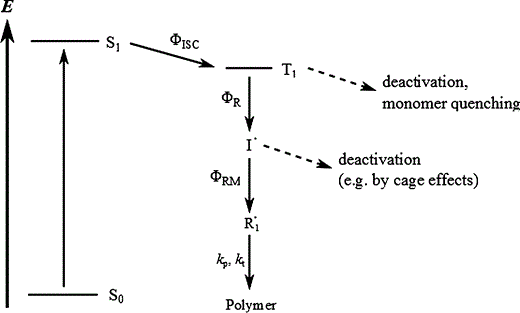
In most cases, absorption will cause the initiator molecule to enter the first singlet excited state, commonly denoted as S1. In general, more than one deactivation channel will be active for the excited species, and not all of them do necessarily lead to free radical generation. The fraction of the excited molecules that are actually converted into primary radicals is expressed by the overall quatum yield Φ, which is the product of the quantum yields of three successive elementary processes:
intersystem crossing from the lowest excited singlet to the lowest triplet state,
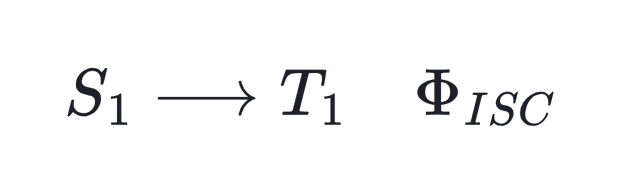
bond scission in the triplet state,
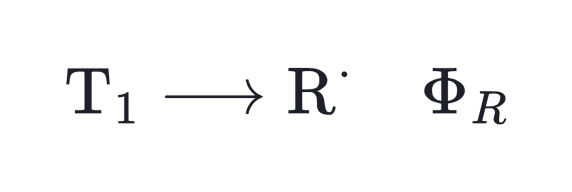
and reaction of the formed radical with a monomer molecule.

For ketones, the first step (intersystem crossing) is typically very efficient (ΦISC≈1), so the quantum yield mainly depends on the second and third steps. Competing processes, such as reaction with oxygen or quenching by monomers, can reduce Φ. This is why experiments often require degassing the reaction mixture.
2.3 Self-initiation
It is not strictly necessary for a radical polymerization to be started by an initiator. It might also be initiated by impurities, by peroxy compounds that are formed in the presence of molecular oxygen, or even by the monomer itself. A prominent example for the latter is the self-initiation of styrene, which proceeds via Diels–Alder reaction of two monomers, as depicted in the scheme below. Such self-initiated polymerization processes are typically limited to elevated temperatures, and can often be prevented under very pure conditions. Few monomers are capable of self-initiation even under very pure conditions, one of which is styrene, reacting via a self Diels–Alder cycloaddition mechanism. The self-initiated bulk polymerization of styrene has a substantial activation energy: a 50% monomer conversion needs 400 days at 29 °C, but only 4 h at 127 °C. However, the produced polystyrene is very pure due to the absence of initiators and other additives.
2.4 Redox-initiation
Redox-initiation is most frequently used in polymerizations in aqueous systems but may be used in organic solvents as well. A redox-initiator consists of an oxidizing and a reducing agent. In most redox initiators, the redox reaction leads to the formation of only one radical, avoiding cage termination processes and thus enhancing the initiator efficiency. For example, Fe2+-peroxide system(Fenton Reaction) is a classical redox-initiation.